 “‘We grow what we can’t engineer, and we engineer what we can’t grow,’” says UCSF bioengineer Shuvo Roy, PhD.
“‘We grow what we can’t engineer, and we engineer what we can’t grow,’” says UCSF bioengineer Shuvo Roy, PhD.
Roy and William Fissell, MD, a nephrologist at Vanderbilt University Medical Center in Nashville, lead a team of 45 collaborators in pursuing a new treatment for end-stage renal disease (ESRD), a condition which affects more than 650,000 people in the United States. Currently the only two treatment options are kidney transplantation, which is limited by a shortage of donor kidneys, and dialysis, which usually requires three lengthy visits a week to a medical facility to filter the patient’s blood of toxins.
Roy and Fissell lead The Kidney Project in developing a third alternative: an implantable bioartificial kidney. This device, which is the size of a coffee cup and is fully implantable, combines design elements inspired by nature with others that directly incorporate human cells.
The device uses a two-stage approach to clean the blood of patients whose own kidneys are no longer up to the job. First, the device uses the power of a patient’s own heart to pump blood through a filter to separate the toxins, producing a watery ultrafiltrate. This ultrafiltrate then cycles through a bioreactor filled with renal tubule cells, which reabsorb electrolytes and most of the water and send toxins and excess water to the bladder.
An Exquisite Sieve
The first stage uses a hemofilter made from silicon nanopore membranes with tiny filtering pores – an exquisitely fine sieve. Taking a page from nature’s design playbook, the holes in the silicon nanopore membranes are not circular, but rather are slit pores – similar to the design of a baleen whale’s food filtering system, which has evolved to use long, narrow slits like teeth on a comb to separate tiny krill from ocean water. “The silicon nanopore membranes are so efficient that they can filtrate blood at very low energy, powered by a patient’s own blood pressure – they do not need a mechanical pump,” said Roy.
Part of the challenge is working at such an incredibly tiny scale. While the semiconductor electronics industry has developed electron beam lithography and focused ion beam etching to carve out components on silicon wafers at the sub-ten nanometer scale, these advanced technologies must be employed in an artisanal manner to make just a few miniscule features. (Ten nanometers is approximately 1/10,000th the width of a human hair.)
That approach is far too slow and expensive to make the millions of pores required by the bioartificial kidney’s hemofilter. So Roy’s team developed a new nanofabrication process. “We came up with a way to use standard optical lithography and etching techniques, applying them in a different way to create sub-ten nanometer pores in a reliable, scalable manner for production,” said Roy.
“Another difference between silicon electronics chips and our device is that most electronics that are implanted in the human body are hermetically sealed, like a pacemaker,” said Roy. “We, on the other hand, are exposing silicon to flowing blood as well as the heart’s pulsatility beating on it 50 to 60 times a minute. But silicon has someattractive properties – it is fatigue resistant, and its surface can be modified with the chemistries and and topography that we want. All of these can then be optimized for blood compatibility and filtration.”
One of the major design challenges is ensuring that the hemofilter resists protein absorption, which can clog membrane pores and lead to blood clot formation. There are several film coatings that can be applied, a bit like painting gold leaf onto a canvas, to prevent protein absorption. However, these coatings were not designed to be applied at such a small scale, so Roy’s team honed a method to graft layers that are one or two molecules thick onto the membrane without blocking off the pores.
If You Can’t Beat Them, Enlist Them
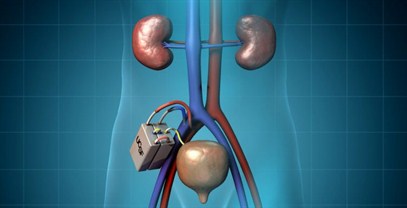
The second part of the bioartificial kidney features a bioreactor, filled with cultured kidney cells from a donor kidney. These renal tubule cells reabsorb most of the useful components – such as salts, sugars and water – back into the bloodstream, while sending waste and excess fluid to the bladder to be excreted as urine.
“The renal tubule cells perform functions that are difficult to engineer,” said Roy. For his team to design a device that could orchestrate the immensely complex task of determining the right concentrations of electrolytes, it would need a myriad of sensors and significant processing power. “The [implanted] cells take care of that naturally, as they’ve been designed to do by evolution,” said Roy.
In collaboration with partners at the University of Michigan, the team harvests renal tubule cells from a donor kidney, culturing them so they can supply multiple bioreactors. Because the kidney cells are encased in silicon membranes, they are isolated from the body’s immune system because white blood cells and other relevant proteins are too big to fit through the nanopores. Therefore, patients will not require immunosuppressant drugs to prevent rejection of implanted cells – a huge advantage, since these medications are often toxic to the kidney and can cost up to $25,000 annually.
The bioreactor’s cells provide additional benefits to patients that dialysis does not. For example, renal tubule cells help with producing hormones, rebalancing the blood pH, and regulating blood pressure. Dialysis patients can receive some but not all of those benefits through medication and lifestyle changes. In addition, dialysis patients are at increased risk of infection because of the need for vascular access, and are tethered to a dialysis machine several times a week. The five-year survival rate for hemodialysis patients is only about 35 percent, compared with about 80 percent of for kidney transplant patients.
The bioartificial kidney filters waste through convection – using the body’s natural blood pressure to separate blood cells, proteins and other larger components from the small toxins and waste products that can fit through the pores of the hemofilter. By contrast, hemodialysis uses a diffusion process to filter the blood, requiring about 120 liters of dialysate liquid per session that is then discarded. In drought-stricken areas like California, this is another advantage of the bioartificial kidney.
The combination of the silicon nanomembrane hemofilter and the bioreactor with human kidney cells brings together the best of both worlds in a biohybrid approach – hence the “bioartificial” kidney, which combines both biological and completely synthetic components.
Surgery and Bioengineering: A Vital Partnership
An idea may look great on paper or function well as a prototype, but all kinds of new questions arise when implanting a device in animals, and eventually in humans in clinical trials. The UCSF Surgical Innovations Program allows surgical residents to spend their two research-focused years working side-by-side with engineers and other innovators to develop and refine new devices.
Surgical resident Willieford Moses, MD worked in Roy’s lab, bringing his complementary skills to the project. “Dr. Moses helped us define the optimal placement and size of the device, and what types of connection we could make between the device and the body,” said Roy.
For example, the engineers had developed a prototype device made of titanium because it is a robust material. When Moses and his clinical colleagues saw it, they pointed out that the heavy device would pull on the blood vessels it was connected to, and recommended that the team consider constructing the device out of a lighter material. Moses also provided practical feedback on the connection between the blood vessels and the device. “Dr. Moses told us, ‘This may be the best flow path you guys designed on the computer, but we really can’t attach it there without causing kinks in the connections, which will cut off the blood supply and cause clots,’” recalled Roy. “With his input, we redesigned the device to make it lighter and to connect it in a much more surgically compatible manner.”
Moses collaborated with experts from a variety of fields to work out the mechanics of connecting the device to blood vessels. “We worked with an engineer to design connector pieces to match the device with commercially available synthetic vascular grafts in order to maintain the blood flow path and to prevent leaking of blood or breakdown of blood products,” said Moses. “We also worked with vascular surgeons to figure out how to connect these synthetic grafts to the device.
“I also headed up in vivo preclinical studies to optimize our device to be hemocompatible,” said Moses. “We worked on figuring out the appropriate level for anticoagulation to maintain [blood] flow through the device and prevent debris buildup, yet not cause any deleterious effects such as bleeding.”
“Having surgical trainees is fantastic, because they can help the engineering team understand how we might change our device to accommodate issues like these,” said Roy. “We can also ask them, ‘How could you change surgical technique to accommodate the constraints of our device?’ It’s a give and take, and by collaborating, we hope to get an answer that’s acceptable to all and beneficial to the patient. That only happens because of surgical trainees working in conjunction with bioengineers, in a setting where everybody is willing to share and build on each other’s expertise.”
Fast-Tracking Innovation
Patients with ESRD make up one percent of the Medicare population, but account for about 7 percent of the Medicare budget. The number of patients with ESRD is increasing by 5 percent annually.
The federal government, which is the primary payer for the medical care of such patients, is encouraging agencies to accelerate technologies that could improve care. The Kidney Project has been selected to participate in two programs sponsored by the US Food and Drug Administration (FDA), the Innovation Pathway 2.0 and the Expedited Access Pathway, both designed to shorten the time required to bring safe and effective devices to market.
“Typically, you never initially ask the FDA for input,” said Roy. “But these programs allow for a real dialogue, where [federal regulators] may say, ‘Shuvo, if you do things in a certain way, it may make things go easier because we’ve seen this work before in other instances,’ without telling us corporate secrets. Or they may tell us to do something, and we can say, ‘We can’t do it – it’s not cost-efficient or technically challenging,’ and then we discuss it. It’s helpful in making sure the device development proceeds in the right direction.”
Part of that dialogue has been mapping out the flow of preclinical tests. “With the FDA, we decided what elements could proceed in parallel, and what had to be sequential, in order to demonstrate safety,” said Roy. “Because we can take some parallel paths, we were able to compress the timeline by at least one-third.” If all goes according to plan and funding is available, Roy hopes to begin first-in-human testing of the hemofilter in late 2017.
While the team’s ultimate goal is to develop a device that is equivalent to a fully functional, healthy kidney, the interim goal is to design a device good enough to provide an alternative to dialysis or transplant. “If you have stage five chronic kidney disease and you don’t get dialysis or transplant, you die,” said Roy. “But if we can develop something that can improve a patient’s condition to stage 3 or 4 so they can get off dialysis, we think that would provide a significant benefit. It’s often the complications from dialysis itself that are the core problem for why dialysis patients have such poor outcomes.
“This is a really unique project, because we hope to provide a therapy for which there is currently no equivalent,” said Roy. “While transplant is completely biological, we are a biohybrid device. What’s made this project possible is collaboration – bringing together bioengineers, surgeons, and other scientists. No one of us could have done this individually.”
by Elizabeth Chur